Where is the most important update of influenza vaccination guidelines this year?
Original pediatric doctor Yan
In order to guide the vaccination before the arrival of influenza season, since 2018, China Center for Disease Control and Prevention has routinely issued the Technical Guide for Influenza Vaccination in China around September every year.
Reading this year’s guide, you will find that the vaccination dose of infants aged 6 months to 35 months has been greatly updated. I hope parents of children of this age will pay attention.
Here, I would like to introduce the changes in the relevant contents of the new and old guides.
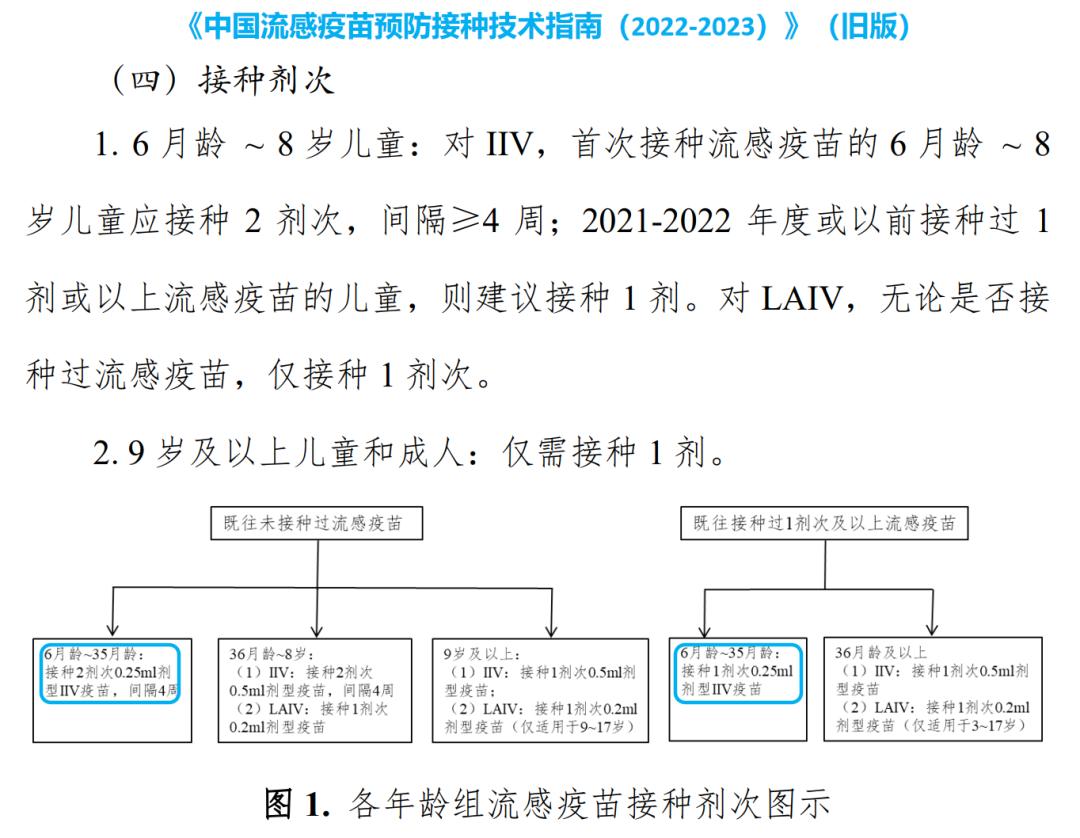
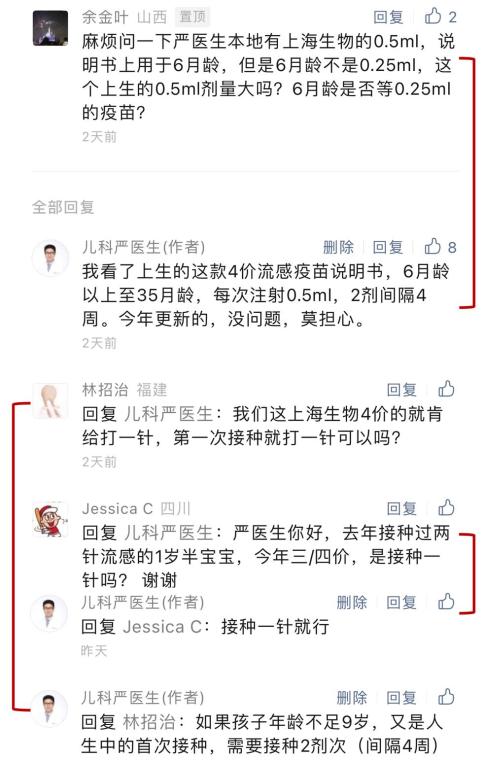
(▲ the old guide of 2022-2023. IIV: inactivated influenza vaccine; LAIV: live attenuated influenza vaccine)
The old guidelines require that children aged 6 months to 35 months should be vaccinated with inactivated influenza vaccine at a dose of 0.25ml (half dose).

However, this year’s new guideline (2023-2024) does not mention the vaccination dose in the diagram, and other contents have not changed significantly.

In the schedule of the new guidelines (below), the basic information of different influenza vaccines in China is listed in detail. Among them, the tetravalent influenza virus split vaccine produced by Shanghai Institute of Biological Products and Shenzhen Sanofi Pasteur Bio requires 0.5 ml (full dose) for people over 6 months old.
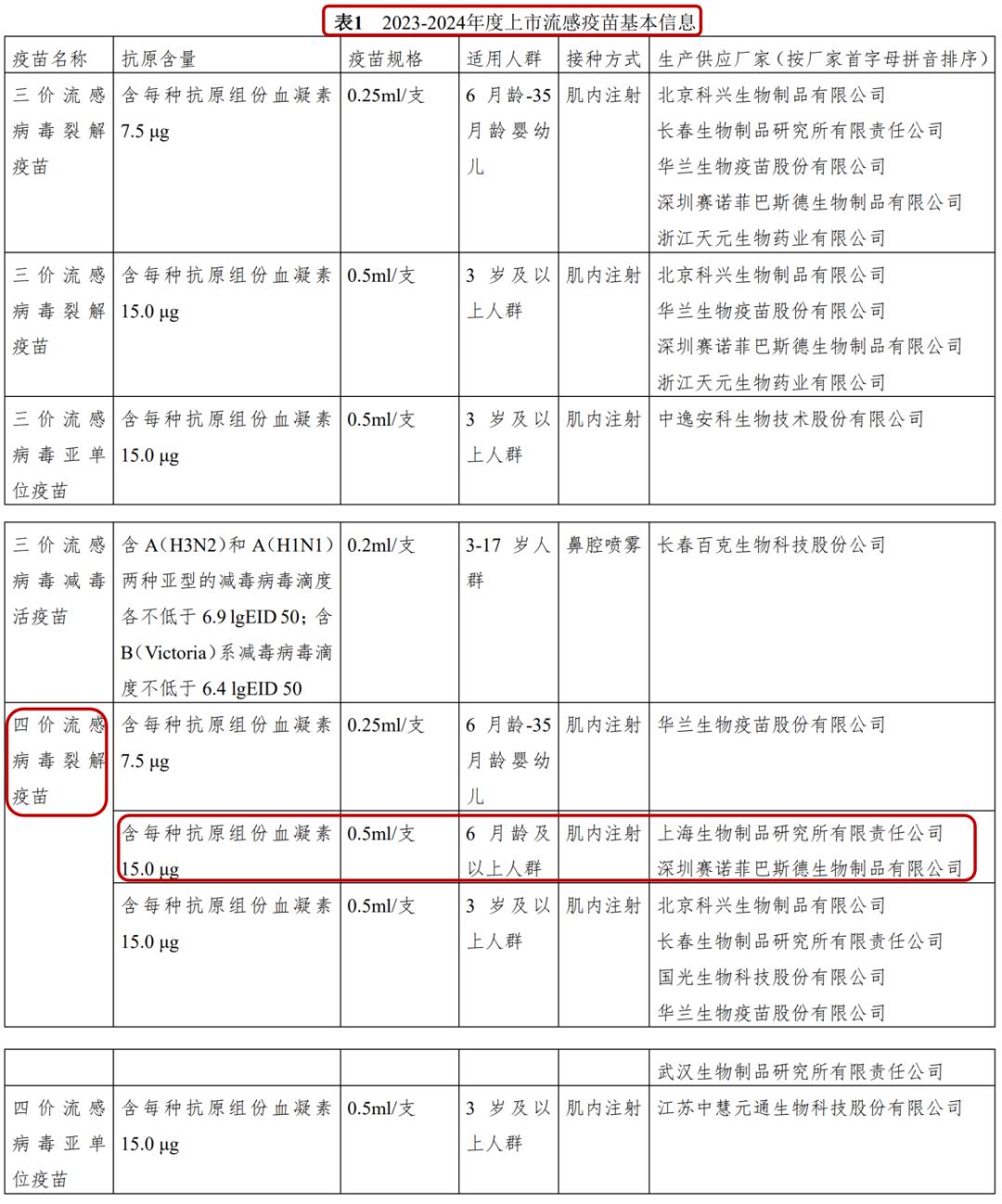
That is to say, if the children are between 6 months and 35 months old, if they are vaccinated with tetravalent influenza virus split vaccines from these two manufacturers this year, they will be vaccinated with 0.5 ml at a time; If it is the first time in his life to get the flu vaccine, he needs to get 2 doses (4 weeks apart).
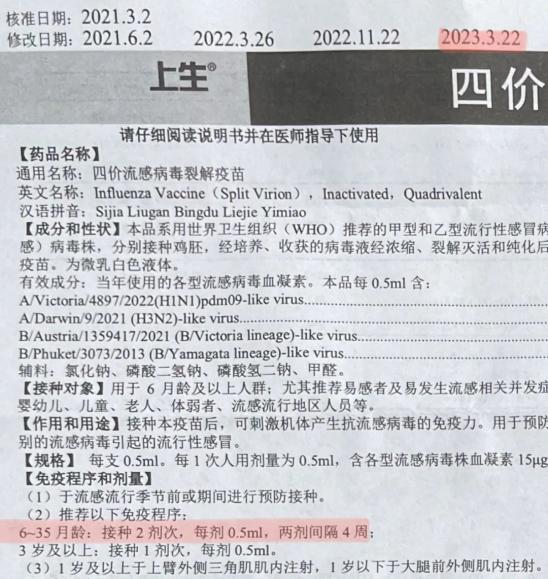
(▲ Part of the instructions for the tetravalent split vaccine produced by Shanghai Institute of Biological Products, which I inoculated myself)
In fact, the whole dose (0.5ml) inactivated influenza vaccine for people aged from 6 months to 35 months has been used in the United States, the European Union, Britain, Canada and other countries, as well as China and Hong Kong.
Multi-country and multi-center clinical trials, including China, show that the immune response of full dose (0.5ml) of Ivv4 to all antigens is better than that of half dose (0.25ml) of Ivv4, and it may improve the protective efficacy of younger children against influenza B..
The 2023-2024 recommendations for prevention and control of influenza in children issued by the American Academy of Pediatrics also mentioned that full-dose IIV4 can be used for people over 6 months old.

(▲ Page 26 of the new guide for 2023-2024)
Therefore, this year, the new guidelines of China CDC also keep pace with the times, suggesting that people aged from 6 months to 35 months can be vaccinated with a full dose (0.5 ml) of tetravalent inactivated influenza vaccine, but it is still limited to the tetravalent influenza virus split vaccine produced by Shanghai Institute of Biological Products and Shenzhen Sanofi Pasteur Bio.
Original title: "Where is the most important update of this year’s influenza vaccination guide? 》
Read the original text