Frontier field of cell therapy: TCR-T cell therapy
foreword
At present, tumor immunotherapy is in the ascendant, and one of the most effective treatment strategies is adoptive cell transfer therapy (ACT). Chimeric antigen receptor (CAR) and engineered T cell receptor (TCR) are the main adoptive T cell immunotherapy in recent years. TCR engineering T cells express tumor antigen-specific receptors, and their α chains and β chains are produced by high-quality and high-affinity antigen-specific T cell clones.
TCR molecule belongs to a superfamily of immunoglobulin, which consists of two covalently bound polymorphic subunits, each of which is antigen-specific, and they are related to at least four different types of signal transduction chains. In order to activate T cells, there must be interaction between TCR and MHC.
The interaction between TCRs and pMHC(peptide-MHC) determines the fate of immature thymocytes, which is very important for the survival of naive T cells. Therefore, TCR-T immunotherapy technology activates the host’s immune system through effective interaction with MHC, especially class II molecules, which are specifically recognized by TCR-T cells and CAR-T cells. TCR-T cells can recognize tumor-specific antigens in cells, while CAR-T cells mainly recognize tumor-specific antigens on the surface. This makes TCR-T cells more effective in tumor treatment.
At present, some new technologies and tools are being applied to TCR-T, which is helpful to improve the efficacy and safety of TCR-T therapy. TCR-T cell therapy is showing great potential for anti-tumor therapy.
Comparison between CAR-T and TCR-T
In ACT therapy, TCR-T and CAR-T cells have been successfully used in the clinical treatment of solid tumors.
CAR contains single chain antibody targeting tumor antigen, transmembrane domain and intracellular activation domain of CD3ζ. In this way, the engineered CAR can recognize specific tumor-associated antigens, and CAR can bind untreated tumor surface antigens without MHC treatment.
The first generation of CAR-T cells showed limited expansion and relatively short persistence, and the "second generation" CAR-T added costimulatory receptors CD28, 4-1BB/CD137 and OX40. These costimulatory receptors are added to the CD3ζ domain of CAR-T cells, thus promoting a more powerful and lasting T cell response. The third generation CARs combines two co-stimulatory signals (CD28 and 4-1BB) at the same time, which has better amplification and longer persistence than the second generation CARs.
On the contrary, TCR is an α/β heterodimer that binds to MHC antigen complex. Compared with TCR, CARs has some disadvantages in identifying tumor antigens, such as extratumoral toxicity. Compared with CARs, TCRs has some structural advantages in T cell-based therapy, such as more subunits (10: 1) in its receptor structure, more tyrosine-based activation motifs (ITAMs) in immune receptors (10: 3), less dependence on antigens (1: 100), and more costimulatory receptors (CD3, CD4, CD28, etc.). TCR with a low MHC affinity range (104-106M-1) can effectively activate T cells, whereas CARs needs a higher affinity range (106-109M-1).
Therefore, CAR-mediated cytotoxicity depends on higher density of cell surface antigens. In addition, T-cell/antigen interaction IS initiated in the structure of immune synapse (IS), in which TCR presents an annular region with peripheral LFA-1 adhesion, while CAR presents a diffuse LFA-1 distribution without annular region. Therefore, TCR-IS is slower but lasts longer than CAR-IS. At the same time, CAR-T cells show faster killing function and migrate to the next tumor target (serial killing), which is in sharp contrast to TCR-T cells’ prolonged signal transduction and killing time.
Recombination TCRs
TCR is one of the most complex receptors in human body. It contains six different receptor subunits, which have a very wide range of functions in T cells. The change of TCR of tumor infiltrating lymphocytes (TILs) significantly affects tumor-specific T cells. Among them, the change of TCR is helpful to the proliferation of T cells, and the diversity of TCR is related to the anti-tumor effect.
TCR engineering of TIL is one of the best treatment methods for tumors. TCR consists of α chain and β chain bound to peptide -MHC ligand, signal subunits (,γ and δ) of CD3 complex and CD3ζ homodimer. Except CD3ζ, all subunits have extracellular immunoglobulin (Ig) domain. Based on these structures, new technologies using engineering TCR include ImmTAC, TRuCs and TAC.
Immune mobilization monoclonal t cell receptor (ImmTAC)
ImmTACs is designed using engineered, soluble and affinity-enhanced monoclonal TCRs(mTCRs). ImmTACs is basically a fusion protein, which combines the engineering TCR targeting system and single chain antibody fragment (scFv) effector function. In the construction of ImmTACs, TCR can recognize peptides from intracellular targets presented by human leukocyte antigen (HLA).
ImmTAC specifically targets HLA- peptide complexes on the surface of tumor cells, and promotes T cell-mediated effector function through the interaction between scFv antibody fragments and CD3. ImmTAC also activates CD8+T cells in a dose-dependent manner, and can effectively redirect and activate CD8+ and CD4+ cells. ImmTAC shows multifunctional reaction by secreting various cytokines, such as TNF-α, IFN-γ, IL-6, MIP1α-β and IFN-γ inducible protein 10.
In addition, selecting the appropriate target antigen is the key to ImmTACs, and mass spectrometry and MHC polymer technology are helpful to identify the appropriate antigen. It is worth noting that TCR engineered T cells also show unexpected targeted toxicity. Generally speaking, ImmTACs has been proved to enhance the anti-tumor response of TCR-T cells, but its safety needs further study.
T cell receptor fusion structure
T cell receptor fusion structure (TRuCs), an antibody binding domain fused with T cell receptor subunits, is designed to effectively recognize tumor surface antigens. TRuCs is composed of specific antibodies targeting tumor-associated antigens fused to the extracellular N- terminal of five TCR subunits (TCRα, TCRβ, CD3, CD3γ and CD3δ), which provides new targeting specificity and HLA-independent target cell clearance for engineered T cells and can be activated by corresponding target cells.
Compared with the second generation of CAR-T cells, this method shows better anti-tumor effect. In addition, TRuCs dominates all the signal mechanisms of TCR complex, while CARs only uses the limited signal of the isolated intracellular segment of CD3ζ.
T cell antigen coupling agent (TAC)
T cell antigen coupling agent is another engineered TCR cell, which can induce more effective anti-tumor response and reduce toxicity in an MHC-independent way. TAC chimeric protein can form TCR/CD3 complex and get more T cell responses by binding to CD3 domain.
The activity of TAC receptor is closely related to the selection of CD3 binding domain. For example, a single chain antibody from OKT3(muromonab-CD3) has lower cytokine production and cytotoxicity compared with UCHT1, which may lead to substantially different functional results. Compared with the second generation of CARs, T cells engineered with TAC gene are not only beneficial to the greater infiltration in solid tumors after adoption, but also reduce the expansion and extratumoral toxicity of T cells in healthy tissues expressing antigens.
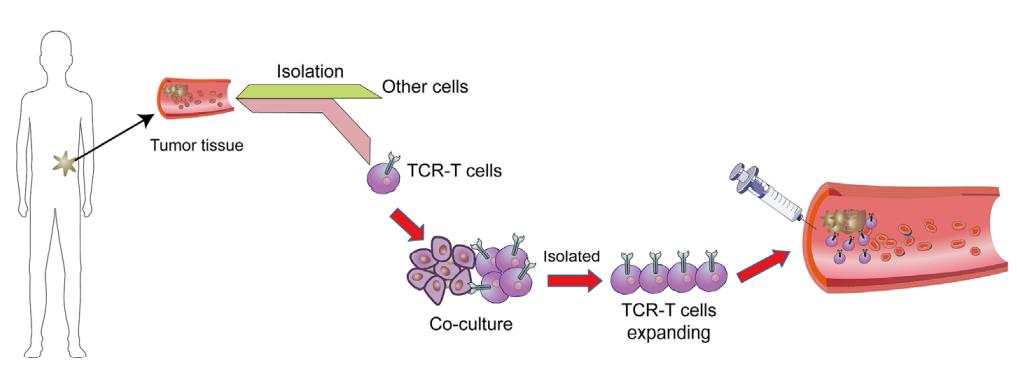
Workflow of TCR-T cell therapy
In order to isolate therapeutic TCR, antigen-specific T cells must be isolated from the blood of patients or healthy blood donors and amplified in vitro with specific peptide antigens and cytokines (such as IL-2 and IL-15). This process needs to determine the specific tumor-related peptide targets that can be safely targeted to patients in advance. After selecting the target antigen, different methods can be used to screen TCR with the required high affinity and tumor specificity. Pre-clinical safety testing is also necessary to ensure the minimum off-target effect and cross-reactivity of isolated high affinity TCR. Viral vectors are usually used to genetically modify autologous patient T cells to express the proven therapeutic TCR, and then transfuse it back into patients.
Determine the target antigen
Melanoma antigen 1(MART-1) recognized by T cells is the first tumor-related antigen targeted in TCR-T clinical trials. After this breakthrough, TCR-T therapies for various tumor antigens have been developed, including TCR-T therapies for MAGE-A3, MAGE-A4, GD2, mesothelin, gp100, MART1, AFP, CEA, NY-ESO-1 and viral peptides derived from HPV and EBV. Among them, NY-ESO-1 has been proved to be one of the most promising targets of TCR-T cells, and it has achieved success in the treatment of synovial sarcoma, with an objective effective rate of 67%.
The ideal TCR-T target antigen shows the following characteristics: (1) the ability to induce immune response; (2) It is related to driving tumor phenotype (such as oncogene) to reduce the risk of antigen loss and tumor immune escape; And (3) expression on tumor stem cells to promote permanent tumor eradication.
Identification method of tumor-associated antigen
High-resolution mass spectrometry (MS) has been proved to be the most powerful Qualcomm method to promote the direct identification of HLA-I binding peptides from tumor cells. In this method, HLA-I/ peptide complexes are separated from tumor tissues or cell lines by immunoprecipitation (IP), and then the binding peptide antigens are separated from HLA-I molecules and antibodies for IP by fully washing and applying acidic elution buffer. This strategy allows each tumor sample to identify thousands of validated peptide targets, and has been used to identify HLA-I ligands of glioblastoma (GB), melanoma, renal cell carcinoma (RCC) and colorectal cancer (CRC).
Identification method of new tumor antigen
Although MS-based technology can be used to identify new antigens, it is more difficult to identify them, especially for tumor samples with limited size, because of their relatively low abundance and limited sensitivity of MS. However, the development of new generation sequencing technology is helpful to identify and locate such tumor targets. All-exon DNA sequencing, combined with computational prediction algorithm, allows to identify specific genetic changes in cancer cells, which can produce mutant peptides and present them on tumor HLA-I molecules.
All somatic mutant genes can be analyzed by computer to predict the potential high affinity epitopes that may bind to HLA-I molecules of patients, so as to be recognized by T cells. With the use of large MS elution peptide database, HLA-I peptide binding prediction algorithm is constantly updated and improved, and other prediction algorithms try to consider biological variables related to the complexity of intracellular processes.
Another commonly used method is tumor RNA sequencing, which allows the selection of new antigens with the highest transcriptional expression. It is worth noting that although these prediction methods usually show very good accuracy in identifying presented and highly immunogenic new antigens, the number of new antigen targets they usually predict is 1 to 2 orders of magnitude higher than the actual number of real targets.
Discovering new antigens by trogocytosis is a new method in recent years. Trogocytosis is a biological phenomenon in the process of cell combination, in which cells share and transfer membrane and membrane-related proteins. Li et al. found that T cell membrane proteins were specifically transferred to tumor target cells, which presented homologous HLA-I/ peptide complexes. Using these T cell-target cell interactions, they created a new antigen discovery system by incubating T cells expressing orphan TCR with homologous target cells. By transferring fluorescent markers from T cells to target cells, this method can isolate these target cells and sequence homologous TCR ligands, thus establishing a new antigen library.
Isolation of tumor-specific T cells and TCR
Using HLA-I polymer, single cell TCR sequencing or antigen-negative humanized mice, tumor-reactive T cells and TCR can be identified from autologous, allogeneic or xenogeneic cell banks.
Using HLA-I multimer method, antigen-specific CD8+T cells can be directly separated by multimer staining and flow cytometry. Before isolating the paired full-length TCR sequences, these polyclonal T cells were tested for homologous peptide recognition and anti-tumor function. TCR with high affinity and specificity can be obtained by using a highly sensitive PCR-based single cell TCR analysis method (TCR-SCAN).
Another method uses humanized mouse TCR gene bank, which will not cause T cell clone deletion or tolerance in humans. Therefore, Li et al. used the whole human TCRα/β gene locus and chimeric HLA-A2 transgene to construct transgenic mice to isolate human TCR against human TAA.
Single cell sequencing method represents a more promising method to isolate tumor-specific TCR coding genes by Qualcomm method. Using RNA bait libraries targeting each individual V and J element in TCRα and TCRβ loci, TCR coding genomic elements can be selectively isolated from excised genomic DNA(gDNA) fragments for subsequent paired terminal deep sequencing. This makes it possible to identify antigen-specific TCR from human materials or oligoclonal T cell populations of TCR humanized mice.
Infantile T cells can also be used as TCR sources for TCR-T therapy. TAA and new antigen-specific T cells can be derived and amplified from low-frequency precursors in peripheral blood of cancer patients, and can be reinfused or used as the source of antigen-specific TCR. Because cancer patients usually show immunosuppression or dominant T cell tolerance, the original sequence of HLA-I matched healthy donors also represents a reliable source, because it has a huge diversity of TCR sequences, and theoretically T cells have any antigen specificity, including new tumor antigens. A Qualcomm-quantity technology platform has been developed to find the original sequence, so as to quickly and effectively identify the rare but valuable TCR for personalized adoptive T cell therapy.
Cloning of TCR
Most TCR-based gene therapy methods rely on in vitro transduction of T cells with viral vectors, and adenovirus is the earliest vector used for gene therapy. However, due to their inability to integrate transgenes into the host genome, TCR expression will be lost during T cell proliferation. In addition, the immune genetic characteristics of adenovirus also limit its application as a gene therapy vector. In contrast, retroviruses show greater prospects as gene transfer vectors, because they can infect a variety of cells and have the ability to insert transgenes into the host genome, thus making the ectopic TCRα/β chain stably expressed.
Retroviral vectors derived from γ -retroviruses such as mouse leukemia virus (MLV) have been widely used for gene transfer into human T cells. This method has been used to transfer various genes, including suicide gene, TCR and CARs. The main disadvantage is that they cannot transduce non-proliferative target cells, which excludes the application of resting T cells in TCR-T therapy. In addition, retrovirus insertion mutation may cause potential side effects.
Recently, lentiviral vectors (LV) have attracted more attention as gene transfer vectors, because they can transfer genes into mitotic and non-splinter cell. Various techniques, such as Golden Gate cloning and LR cloning, are usually used to construct a vector for inserting TCRα/β gene.
Adeno-associated virus (AAV) is another widely used virus vector. Compared with adenovirus vector, AAV has lower immunogenicity and wider cell tropism, so it has been widely used in tumor gene therapy. In order to promote the integration of transgenes, people have developed self-complementary AAV vector (scAAV) to make AAV independent of the complementary strand synthesis of host cells. scAAV is more effective than traditional AAV in preclinical model.
At the same time, some non-viral gene editing methods have also been developed. MRNA electroporation has been proved to achieve transient expression of TCR and CAR, thus minimizing the risk of persistent virus components. Clinical data show that both TCR-T and CAR T cells modified by mRNA are feasible and safe, and there is no obvious evidence that they have non-targeted toxicity to normal tissues. However, the lack of continuous TCR expression may limit the curative effect and require repeated infusion. In addition, the non-viral sleeping beauty retrotransposon system is also used for the transduction of TCR and CARs.
Gene editing can insert large gene fragments into target cells specifically and efficiently through homologous directional repair (HDR). TCR-T cells developed by CRISPR/CAS9 have been proved to specifically recognize tumor antigens in vitro and induce a productive anti-tumor response in vivo.
Verification method of TCR
After TCR cloning, extensive preclinical verification is needed to prove the specificity and safety of engineered TCR-T cells. Verification includes evaluating the affinity of TCR by titration of homologous peptide antigens and measuring the killing effect of a group of HLA-I matched tumor cell lines. If there is no such tumor cell line, the target cells can transductively express related antigens and related HLA-I molecules. The new antigen can also be expressed in autologous antigen presenting cells to evaluate the antigen reactivity of TCR.
The safety test includes testing the ability of candidate TCR-T to identify HLA-I matching primary tissues, so as to ensure that there is no normal tissue as the target, which may lead to non-targeted toxicity. In at least two clinical trials of TCR-T cell therapy, there was a cross reaction between normal brain cells and heart cells, which led to the death of patients. These test results emphasize the importance of extensive safety tests before TCR enters clinical trials.
Safety of TCR-T
The ACT of TCR-T cells showed high tumor killing, but some serious adverse events also appeared in some clinical studies. It is very important to optimize TCR affinity in engineered T cells, and receptor affinity can determine the safety and effectiveness of T cell therapy. In terms of curative effect, affinity TCR interaction is enough to activate T cells, but strong affinity is needed to maintain the expansion of T cells.
In phase I/II ACT clinical trials, engineered T cells with low affinity show safer characteristics, but their anti-tumor response is weak. By identifying the TCR-pMHC interaction of T cells, engineered T cells can be divided into high affinity type and low affinity type. In addition, some technologies have been developed to improve the safety of TCR-T.
The safety switch mechanism based on engineered T cells is an attractive strategy. Thymidine kinase gene from herpes simplex virus type I (HSV-TK) is one of the most common suicide genes.
Although HSV-TK shows safety in cell-based immunotherapy, it is necessary to introduce phosphorylated nucleoside analogues. Another safer induced T cell safety switch is called inducible caspase-9(iC9). IC9 is a modified human FK binding protein, which can be activated by a small molecule compound AP1903, and this process depends on the mitochondrial apoptosis pathway.
The immunogenicity of iC9 suicide gene is low, and the immune response to transgenic cells is reduced. IC9-based safety switch has been proved to be more potential for cell therapy than previous suicide genes.
Clinical status of TCR-T cell therapy
As of August 9, 2021, there are 175 studies using TCR-T therapy in ClinicalTrials, 71 of which are specific TCR for specific TAA or new antigen, and 32 studies have been completed. NY-ESO-1 is the most common targeted antigen, which is expressed in many cancers, including myeloma and melanoma. Other tumor testis-associated antigens, such as PRAME and MAGE proteins, melanoma differentiation antigens MART-1 and gp100, and recent cancer drivers, such as WT1, KRAS and TP53, are also popular TCR-T targets.
A total of 83 sponsors/collaborators initiated or participated in the research of TCR-T cell therapy, including the National Institutes of Health (NIH), government organizations, industries and universities/academic institutions. At present, the National Cancer Institute (NCI) has supported 53 TCR-T projects, accounting for 20% of all ongoing projects.
Among the 29 pharmaceutical companies developing TCR-T therapy, GlaxoSmithKline and Adaptimunime initiated the most clinical trials, with 11 and 7 respectively. Recently, a phase 1 clinical trial (NCT02858310) of TCR-T cells targeting human papillomavirus (HPV)-16 E7 protein in the treatment of metastatic human papillomavirus-related epithelial cancer was reported. In this study, 6 of the 12 patients who received treatment showed objective clinical response and observed steady tumor regression. This is a landmark clinical trial of TCR-T cell therapy, which proves that targeted virus antigen has good clinical effect on patients with virus-related cancer. Other viral antigens explored as TCR targets include HPV-E6 protein, antigens from Epstein-Barr virus (EBV) and human endogenous retrovirus (HERV) targets, such as Herv-E.
MART-1 and NY-ESO-1 TCR-T therapy targeting TAAs also showed clinical efficacy in advanced melanoma, myeloma and non-small cell lung cancer. The total effective rate (ORR) of TCR-T clinical trial is between 0 and 60%. It is worth noting that most of these TCR-T clinical trials only enrolled a small number of patients (2 to 25), so ORR may not be statistically accurate. Therefore, larger-scale phase II and III clinical trials are needed to confirm the actual clinical efficacy of these TCR-T therapies.
Challenges and potential solutions of TCR-T cell therapy
Although immunotherapy based on TCR-T cells has shown certain clinical efficacy in most patients receiving treatment, it still faces many challenges in many fields. These challenges include: (1) Immunotoxicity caused by targeting normal tissues; (2) The expression of TCR in engineered T cells is insufficient or transient; (3)T cell exhaustion and dysfunction; (4) tumor immune escape, and (5) most cancer patients lack effective tumor-specific antigens as targets. Overcoming these challenges will be the key to greater clinical success in the future.
Discovery of new targets
At present, the target of peptide antigen for effective and safe immunotherapy of TCR-T is very limited. At present, most of the targets used are TAA. Although it is up-regulated in tumor tissues, it still maintains a low level of expression in normal tissues, which may lead to autoimmune toxicity. Therefore, the new antigen seems to be the safest target for TCR-T cancer treatment. However, the main challenges of developing new antigens in TCR-T clinic include: (1) the mutation of new antigens is individualized to a great extent, and there are differences among cancer patients, so it is difficult to develop widely used immunotherapy products; (2) The expression of new antigens in tumor tissues is often heterogeneous.
Nevertheless, recent reports have highlighted the emergence of new immunogenic antigens widely shared by tumor cells, including mutated KRAS and TP53. Many other studies have also demonstrated the immunogenicity of shared new antigens that can be used to generate potential therapeutic tumor-specific TCR. With the development of next generation sequencing technology, especially single cell DNA sequencing, transcriptome sequencing and mature in vitro verification methods, TCR-T immunotherapy targeting personalized new antigens may become a popular cancer treatment method in the next few years. In addition, the emerging TAA class, such as carcinoembryonic antigen, may also constitute a feasible target for the future development of TCR-T.
Maximize therapeutic TCR expression
The correct pairing of transgenic α and β chains is one of the main challenges that hinder the development of TCR-T cells. Since each transduced T cell includes two endogenous TCR chains and two transformed TCR chains, heterodimers with unknown specificity can lead to potential autoimmune consequences. Another related problem is that improper α/β chain TCR pairing will compete for CD3 complex, thus reducing the surface expression and signal transduction of therapeutic TCR.
There are several methods to properly pair the transduced TCR chains, including: (1) the constant region of partially murine TCR; (2) adding cysteine residues to promote the introduction of disulfide bonds in TCR chains; (3) changing the secondary structure of the constant region of endogenous TCR; (4) adding a signal domain to the intracellular part transduced with TCR; (5) Introducing TCR-α/β chain into substitute effector cells or constructing single-stranded TCR.
Methods to enhance the expression of therapeutic TCR include: (1) codon optimization of TCR-α and TCR-β chain transgene, and (2) changing the configuration of TCR-α/TCR-β vector to optimize expression.
Reduce adverse events
Usually, targeting non-tumor toxicity is the main key obstacle of TAA, and this risk urges researchers to study common new antigens more carefully. At present, several oncogene hot spot mutations are being studied as potential TCR targets, such as phosphoinositide -3- kinase (PI3K), KRAS and TP53. In addition, genetically engineered TCR-T cells with suicide genes are an important safety measure. Obviously, it is very important to develop reliable, identifiable, personalized, highly specific and immunogenic tumor antigen targets to reduce adverse events related to TCR-T cell therapy.
Graft-versus-host disease of allogeneic T cells
Using allogeneic T cells is a very promising scheme, which can overcome manufacturing problems, patient-related immune cell defects and treatment delays. In order to use allogeneic T cells, it is necessary to control graft-versus-host disease caused by transduced allogeneic reactive lymphocytes and rejection of engineered lymphocytes by host immune system.
The deletion of endogenous TCR gene, HLA-I locus or CD52 molecule is one of the strategies to avoid the failure of TCR-T transplantation, which can be achieved by many methods, such as gene editing or using siRNA. In addition, pluripotent stem cell technology is also considered as a potential solution.
summarize
In recent years, engineered T cells have shown excellent curative effect in the treatment of hematological tumors. TCR regulation is very important for T cell reactivation, immune response and its clinical effect on foreign antigens. TCR-T cells have incomparable advantages over CAR-T cells, and show great potential in preclinical and clinical research.
However, there are still several key challenges to improve the anti-tumor efficacy of TCR-T immunotherapy, including how to safely increase the affinity of therapeutic TCR, how to identify the common tumor-specific antigen and TCR in the patient population, and how to regulate the expression of TCR and achieve the best function. The solution of these problems will help to give full play to the potential of TCR-T cell therapy and bring hope to cancer patients to relieve their pain.
References:
1.Engineered TCR-T CellI mmunotherapy in Anticancer Precision Medicine: Pros and Cons. Front Immunol. 2021; 12: 658753.
2. Evolution of CD8+ T Cell Receptor(TCR) Engineered Therapies for the Treatment of Cancer. Cells. 2021 Sep; 10(9): 2379.